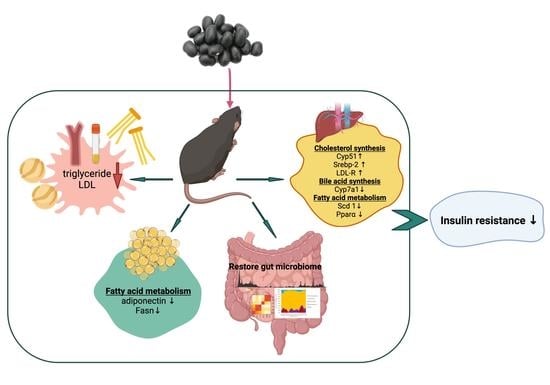
Cooked Black Turtle Beans Ameliorate Insulin Resistance and Restore Gut Microbiota in C57BL/6J Mice on High-Fat Diets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Black Turtle Bean Preparation
2.2. Animals and Diets
2.3. Blood and Tissue Sample Collection
2.4. Blood Glucose, Plasma and Hepatic Lipid Analysis
2.5. Glucose Tolerance Test (GTT) and Area under Curve (AUC)
2.6. Plasma Levels of Diabetes Related Biomarkers and Inflammatory Cytokines
2.7. Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) Index Calculation
2.8. Gut Microbiome Analysis
2.9. RT-PCR Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Metrics of Mice
3.2. Plasma Lipoprotein, Triglyceride Levels and Hepatic Lipid Contents
3.3. Glucose Tolerance Test (GTT) and Insulin Resistance
3.4. Biomarkers Associated with Diabetes and Obesity
3.5. Plasma Levels of Inflammatory Cytokines
3.6. Gut Microbiome Analysis
3.7. RT-PCR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akibode, C.S.; Maredia, M.K. Global and Regional Trends in Production, Trade and Consumption of Food Legume Crops; Michigan State University: East Lansing, MI, USA, 2012; pp. 1–83. [Google Scholar]
- Minor, T.; Bond, J.K. Vegetables and Pulses Outlook; United States Department of Agriculture: Washington, DC, USA, 2018. [Google Scholar]
- Havemeier, S.; Erickson, J.; Slavin, J. Dietary guidance for pulses: The challenge and opportunity to be part of both the vegetable and protein food groups. Ann. N. Y. Acad. Sci. 2017, 1392, 58–66. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.J.; Chang, S. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.; Chang, S.K. Digestive enzyme inhibition activity of the phenolic substances in selected fruits, vegetables and tea as compared to black legumes. J. Funct. Foods 2017, 38, 644–655. [Google Scholar] [CrossRef]
- Hernández-Saavedra, D.; Mendoza-Sánchez, M.; Hernández-Montiel, H.L.; Guzmán-Maldonado, H.S.; Loarca-Piña, G.F.; Salgado, L.M.; Reynoso-Camacho, R. Cooked common beans (Phaseolus vulgaris) protect against β-cell damage in streptozotocin-induced diabetic rats. Plant Foods Hum. Nutr. 2013, 68, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Lepp, D.; Zhang, C.P.; Wu, W.; Zarepoor, L.; Lu, J.T.; Pauls, K.P.; Tsao, R.; Wood, G.A.; Robinson, L.E. Diets enriched with cranberry beans alter the microbiota and mitigate colitis severity and associated inflammation. J. Nutr. Biochem. 2016, 28, 129–139. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; Ni, L.; Vincken, J.-P. Green and black tea phenolics: Bioavailability, transformation by colonic microbiota, and modulation of colonic microbiota. J. Agric. Food Chem. 2018, 66, 8469–8477. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, S.K.C.; Zhang, Y.; Hsu, C.-Y.; Nannapaneni, R. Gut microbiota and short chain fatty acid composition as affected by legume type and processing methods as assessed by simulated in vitro digestion assays. Food Chem. 2020, 312, 126040. [Google Scholar] [CrossRef]
- Bravo, L.; Siddhuraju, P.; Saura-Calixto, F. Effect of various processing methods on the in vitro starch digestibility and resistant starch content of indian pulses. J. Agric. Food Chem. 1998, 46, 4667–4674. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Lanza, E.; Kris-Etherton, P.M.; Colburn, N.H.; Bagshaw, D.; Rovine, M.J.; Ulbrecht, J.S.; Bobe, G.; Chapkin, R.S.; Hartman, T.J. A high legume low glycemic index diet improves serum lipid profiles in men. Lipids 2010, 45, 765–775. [Google Scholar] [CrossRef] [Green Version]
- Carbonaro, M.; Maselli, P.; Nucara, A. Structural aspects of legume proteins and nutraceutical properties. Food Res. Int. 2015, 76, 19–30. [Google Scholar] [CrossRef]
- Lovegrove, A.; Edwards, C.; De Noni, I.; Patel, H.; El, S.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Bartley, G.E.; Arvik, T.; Lipson, R.; Nah, S.-Y.; Seo, K.; Yokoyama, W. Dietary supplementation of chardonnay grape seed flour reduces plasma cholesterol concentration, hepatic steatosis, and abdominal fat content in high-fat diet-induced obese hamsters. J. Agric. Food Chem. 2014, 62, 1919–1925. [Google Scholar] [CrossRef]
- Shao, D.; Bartley, G.E.; Yokoyama, W.; Pan, Z.; Zhang, H.; Zhang, A. Plasma and hepatic cholesterol-lowering effects of tomato pomace, tomato seed oil and defatted tomato seed in hamsters fed with high-fat diets. Food Chem. 2013, 139, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Bowe, J.E.; Franklin, Z.J.; Hauge-Evans, A.C.; King, A.J.; Persaud, S.J.; Jones, P.M. Metabolic phenotyping guidelines: Assessing glucose homeostasis in rodent models. J. Endocrinol. 2014, 222, G13–G25. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L. The Miqe Guidelines: M Inimum i Nformation for Publication of q Uantitative Real-Time pcr e Xperiments; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Kim, H.; Kim, D.-H.; Seo, K.-H.; Chon, J.-W.; Nah, S.-Y.; Bartley, G.E.; Arvik, T.; Lipson, R.; Yokoyama, W. Modulation of the intestinal microbiota is associated with lower plasma cholesterol and weight gain in hamsters fed chardonnay grape seed flour. J. Agric. Food Chem. 2015, 63, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Reverri, E.J.; Randolph, J.M.; Kappagoda, C.T.; Park, E.; Edirisinghe, I.; Burton-Freeman, B.M. Assessing beans as a source of intrinsic fiber on satiety in men and women with metabolic syndrome. Appetite 2017, 118, 75–81. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, A.; Goni, I.; Saura-Calixto, F. Resistant starch and potential glycaemic index of raw and cooked legumes (lentils, chickpeas and beans). Z. Lebensm. Forsch. A 1998, 206, 284–287. [Google Scholar] [CrossRef]
- Mishra, S.; Monro, J.; Hedderley, D. Effect of processing on slowly digestible starch and resistant starch in potato. Starch-Stärke 2008, 60, 500–507. [Google Scholar] [CrossRef]
- Da Silva, C.S.; Haenen, D.; Koopmans, S.J.; Hooiveld, G.J.; Bosch, G.; Bolhuis, J.E.; Kemp, B.; Müller, M.; Gerrits, W.J. Effects of resistant starch on behaviour, satiety-related hormones and metabolites in growing pigs. Animal 2014, 8, 1402–1411. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Jiang, W.; Thompson, H.J. Edible dry bean consumption (phaseolus vulgaris l.) modulates cardiovascular risk factors and diet-induced obesity in rats and mice. Br. J. Nutr. 2012, 108, S66–S73. [Google Scholar] [CrossRef] [Green Version]
- Duane, W. Effects of legume consumption on serum cholesterol, biliary lipids, and sterol metabolism in humans. J. Lipid Res. 1997, 38, 1120–1128. [Google Scholar] [CrossRef]
- Tanaka, M.; Honda, Y.; Miwa, S.; Akahori, R.; Matsumoto, K. Comparison of the effects of roasted and boiled red kidney beans (Phaseolus vulgaris L.) on glucose/lipid metabolism and intestinal immunity in a high-fat diet-induced murine obesity model. J. Food Sci. 2019, 84, 1180–1187. [Google Scholar] [CrossRef]
- Nasteska, D.; Harada, N.; Suzuki, K.; Yamane, S.; Hamasaki, A.; Joo, E.; Iwasaki, K.; Shibue, K.; Harada, T.; Inagaki, N. Chronic reduction of gip secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes 2014, 63, 2332–2343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naitoh, R.; Miyawaki, K.; Harada, N.; Mizunoya, W.; Toyoda, K.; Fushiki, T.; Yamada, Y.; Seino, Y.; Inagaki, N. Inhibition of gip signaling modulates adiponectin levels under high-fat diet in mice. Biochem. Biophys. Res. Commun. 2008, 376, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Heek, M.; Compton, D.S.; France, C.F.; Tedesco, R.P.; Fawzi, A.B.; Graziano, M.P.; Sybertz, E.J.; Strader, C.D.; Davis, H.R. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J. Clin. Investig. 1997, 99, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Muscelli, E.; Natali, A.; Gabriel, R.; Mitrakou, A.; Flyvbjerg, A.; Golay, A.; Hojlund, K. Association of fasting glucagon and proinsulin concentrations with insulin resistance. Diabetologia 2007, 50, 2342–2347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobori, M.; Masumoto, S.; Akimoto, Y.; Oike, H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a western-style diet in c57/bl6j mice. Mol. Nutr. Food Res. 2011, 55, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Jia, Y.; Hu, T.; Du, J.; Wang, Y.; Cheng, B.; Li, K. Gc-(4 → 8)-gcg, a proanthocyanidin dimer from camellia ptilophylla, modulates obesity and adipose tissue inflammation in high-fat diet induced obese mice. Mol. Nutr. Food Res. 2019, 63, 1900082. [Google Scholar] [CrossRef]
- Anitescu, M.; Chace, J.H.; Tuetken, R.; YI, A.-K.; Berg, D.J.; Krieg, A.M.; Cowdery, J.S. Interleukin-10 functions in vitro and in vivo to inhibit bacterial DNA-induced secretion of interleukin-12. J. Interferon Cytokine Res. 1997, 17, 781–788. [Google Scholar] [CrossRef]
- Mito, N.; Hosoda, T.; Kato, C.; Sato, K. Change of cytokine balance in diet-induced obese mice. Metabolism 2000, 49, 1295–1300. [Google Scholar] [CrossRef]
- Lopez-Legarrea, P.; de la Iglesia, R.; Abete, I.; Navas-Carretero, S.; Martinez, J.A.; Zulet, M.A. The protein type within a hypocaloric diet affects obesity-related inflammation: The resmena project. Nutrition 2014, 30, 424–429. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Hashemi, Z.; Fouhse, J.; Im, H.S.; Chan, C.B.; Willing, B.P. Dietary pea fiber supplementation improves glycemia and induces changes in the composition of gut microbiota, serum short chain fatty acid profile and expression of mucins in glucose intolerant rats. Nutrients 2017, 9, 1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, W.; Chin, Y.; Chen, X.; Mi, Y.; Xue, C.; Wang, Y.; Tang, Q. The role of gut microbiota in the resistance to obesity in mice fed a high fat diet. Int. J. Food Sci. Nutr. 2020, 71, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, J.; Zhang, N.; Fu, Y.; Zhang, Z.; Li, Y.; Wang, W.; Li, Y.; Shen, P.; Cao, Y. Ripened pu-erh tea extract protects mice from obesity by modulating gut microbiota composition. J. Agric. Food Chem. 2019, 67, 6978–6994. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.; Yan, B.; Zhao, S.; Zhou, X. Regulation of tartary buckwheat-resistant starch on intestinal microflora in mice fed with high-fat diet. Food Sci. Nutr. 2020, 8, 3243–3251. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhang, Q.; Zhang, Y.; Yao, Z.; Song, P.; Wei, L.; Zhao, G.; Yan, Z. Effect of tartary buckwheat, rutin, and quercetin on lipid metabolism in rats during high dietary fat intake. Food Sci. Nutr. 2020, 8, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, C.; Niu, X.; Zhang, Z.; Li, F.; Li, F. The effects of milk replacer allowance and weaning age on the performance, nutrients digestibility, and ruminal microbiota communities of lambs. Anim. Feed. Sci. Technol. 2019, 257, 114263. [Google Scholar] [CrossRef]
- Sheflin, A.M.; Borresen, E.C.; Kirkwood, J.S.; Boot, C.M.; Whitney, A.K.; Lu, S.; Brown, R.J.; Broeckling, C.D.; Ryan, E.P.; Weir, T.L. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors. Mol. Nutr. Food Res. 2017, 61, 1500905. [Google Scholar] [CrossRef] [Green Version]
- Duca, F.A.; Sakar, Y.; Lepage, P.; Devime, F.; Langelier, B.; Doré, J.; Covasa, M. Replication of obesity and associated signaling pathways through transfer of microbiota from obese-prone rats. Diabetes 2014, 63, 1624–1636. [Google Scholar] [CrossRef] [Green Version]
- Martínez, I.; Wallace, G.; Zhang, C.; Legge, R.; Benson, A.K.; Carr, T.P.; Moriyama, E.N.; Walter, J. Diet-induced metabolic improvements in a hamster model of hypercholesterolemia are strongly linked to alterations of the gut microbiota. Appl. Environ. Microbiol. 2009, 75, 4175–4184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, I.; Perdicaro, D.J.; Brown, A.W.; Hammons, S.; Carden, T.J.; Carr, T.P.; Eskridge, K.M.; Walter, J. Diet-induced alterations of host cholesterol metabolism are likely to affect the gut microbiota composition in hamsters. Appl. Environ. Microbiol. 2013, 79, 516–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Lv, Z.; Zhao, W.; Wang, L.; He, N. Collagen peptide from walleye pollock skin attenuated obesity and modulated gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2020, 74, 104194. [Google Scholar] [CrossRef]
- Bartley, G.E.; Yokoyama, W.; Young, S.A.; Anderson, W.H.; Hung, S.-C.; Albers, D.R.; Langhorst, M.L.; Kim, H. Hypocholesterolemic effects of hydroxypropyl methylcellulose are mediated by altered gene expression in hepatic bile and cholesterol pathways of male hamsters. J. Nutr. 2010, 140, 1255–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. NPJ Biofilms Microbiomes 2019, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Stravitz, R.T.; Dent, P.; Hylemon, P.B. Down-regulation of cholesterol 7α-hydroxylase (cyp7a1) gene expression by bile acids in primary rat hepatocytes is mediated by the c-jun n-terminal kinase pathway. J. Biol. Chem. 2001, 276, 15816–15822. [Google Scholar] [CrossRef] [Green Version]
- Abel, E.D.; Peroni, O.; Kim, J.K.; Kim, Y.-B.; Boss, O.; Hadro, E.; Minnemann, T.; Shulman, G.I.; Kahn, B.B. Adipose-selective targeting of the glut4 gene impairs insulin action in muscle and liver. Nature 2001, 409, 729. [Google Scholar] [CrossRef] [PubMed]







| Ingredient | High-Fat Diet (HF) | High-Fat Diet with Beans (HFB) | Low-Fat Diet (LF) |
|---|---|---|---|
| Lard fat | 225.0 | 225.0 | 63.0 |
| Soybean oil | 25.0 | 23.2 | 7.0 |
| Cholesterol | 0.8 | 0.8 | 0.8 |
| Cellulose | 50.0 | 17.0 | 50.0 |
| 20% beans | - | 200.0 | - |
| Casein | 200.0 | 157.5 | 200.0 |
| Corn Starch | 148.2 | 25.5 | 528.2 |
| Sucrose | 300.0 | 300.0 | 100.0 |
| Cystine | 3.0 | 3.0 | 3.0 |
| Choline Bitartrate | 3.0 | 3.0 | 3.0 |
| Mineral Mix | 35.0 | 35.0 | 35.0 |
| Vitamin Mix | 10.0 | 10.0 | 10.0 |
| Total weight | 1000.0 | 1000.0 | 1000.0 |
| Calories/Kg | 4850.0 | 4839.0 | 3950.0 |
| HF | HFB | LF | |
|---|---|---|---|
| Body weight gain (g) | 12.41 ± 0.82a | 10.56 ± 0.85a | 4.40 ± 0.28b |
| Total energy intake (Kcal) | 707.00 ± 37.8a | 615.00 ± 13.2b | 587.00 ± 20.2b |
| Feed efficiency ratio (g gain/g feed) | 0.09 ± 0.01a | 0.08 ± 0.01a | 0.03 ± 0.01b |
| Feed efficiency ratio (g gain/Kcal feed) | 0.02 ± 0.002a | 0.02 ± 0.001a | 0.01 ± 0.001b |
| Liver weight (g) | 1.02 ± 0.03a | 1.03 ± 0.03a | 0.91 ± 0.05b |
| Liver/body weight | 0.03 ± 0.0003b | 0.03 ± 0.0008ab | 0.03 ± 0.0011a |
| Adipose weight (g) | 0.96 ± 0.07a | 0.80 ± 0.08a | 0.31 ± 0.03b |
| Adipose/body weight | 0.03 ± 0.0016a | 0.02 ± 0.0021a | 0.01 ± 0.0008b |
| Fasting blood glucose (mg/dL) | 157.88 ± 11.21a | 167.75 ± 9.76a | 122.88 ± 8.88b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Tam, C.C.; Meng, S.; Zhang, Y.; Alves, P.; Yokoyama, W. Cooked Black Turtle Beans Ameliorate Insulin Resistance and Restore Gut Microbiota in C57BL/6J Mice on High-Fat Diets. Foods 2021, 10, 1691. https://doi.org/10.3390/foods10081691
Tan Y, Tam CC, Meng S, Zhang Y, Alves P, Yokoyama W. Cooked Black Turtle Beans Ameliorate Insulin Resistance and Restore Gut Microbiota in C57BL/6J Mice on High-Fat Diets. Foods. 2021; 10(8):1691. https://doi.org/10.3390/foods10081691
Chicago/Turabian StyleTan, Yuqing, Christina C. Tam, Shi Meng, Yan Zhang, Priscila Alves, and Wallace Yokoyama. 2021. "Cooked Black Turtle Beans Ameliorate Insulin Resistance and Restore Gut Microbiota in C57BL/6J Mice on High-Fat Diets" Foods 10, no. 8: 1691. https://doi.org/10.3390/foods10081691