
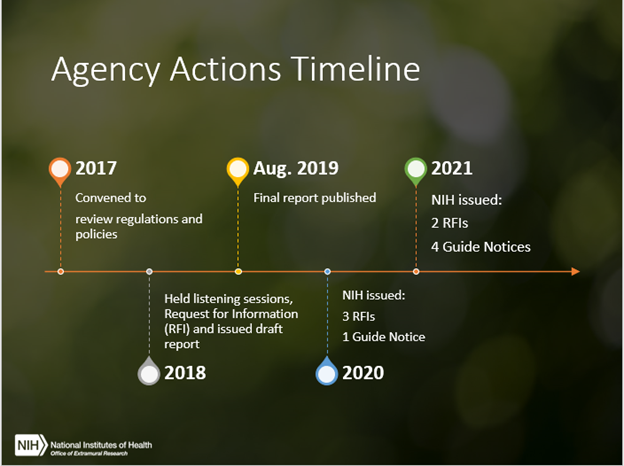
The 21st Century Cures Act called for NIH to collaborate with the U.S. Department of Agriculture (USDA) and Food and Drug Administration (FDA) to reduce administrative burdens associated with laboratory animal research programs, while maintaining high standards of animal welfare as well as the integrity and credibility of the research. We jointly released a final report in 2019 outlining steps to accomplish this goal, and have since worked together to implement many of the recommendations. We wanted to take this opportunity today to share some of NIH’s progress and how you can remain involved.
What we discuss here can also be found on the NIH Office of Laboratory Animal Welfare (OLAW) dedicated 21st Century Cures page. The site and accompanying news feed are regularly updated as new guidance, resources, and related content become available. You can also watch this recently released webinar to learn more.
Let’s look at some of our activities to date:
One of our earliest efforts was harmonizing our annual reporting timeline with that of USDA, simplifying submission timelines for reporting. The reporting period is now October 1 – September 30, which aligns with the federal fiscal year and the USDA’s reporting period (instead of the calendar year). The Annual Report must be submitted to OLAW by December 1.
A list of flexibilities is now available to help institutions conduct facility inspections more efficiently. These flexibilities center around who may conduct the inspections, timing of the inspections, discretion to choose announced or unannounced visits, options for remote inspections, and when and how AAALAC International site visits may be used, among others. We also have a convenient checklist that institutions may use to inspect the animal facilities that includes all the inspection requirements. The checklist is voluntary, and institutions are free to modify it to meet their specific needs.
We also clarified the institutional requirements, responsibilities, timing, and conduct of congruence reviews between grants/contracts and animal protocols. Congruence reviews help ensure that what the Institutional Animal Care and Use Committee (IACUC) approves is consistent with what is in either the application or proposal. These reviews may also help identify potentially serious discrepancies between the grant or contract and approved animal protocol.
OLAW has hosted various virtual trainings to assist institutions to optimize animal welfare, enhance communication with investigators, and recognize pitfalls that increase unnecessary administrative burden. Examples include:
- Webinars and associated materials addressing progress towards implementing the 21st Century Cures Act (see the “Policy & Regulation” topic area on OLAW’s website)
- Offerings as part of the Interagency Collaborative Animal Research Education (ICARE) Project (e.g., ICARE Dialogues and OLAW Conversations) that take place in informal settings where attendees can ask questions about implementing 21st Century Cures initiatives
Coordinated efforts to reduce burden take time. Various federal agencies have an interest in laboratory animal welfare, but each agency has individual requirements, policies, and regulations. As such, in addition to USDA and FDA, NIH now meets twice a year with many other federal agencies to discuss actions that help harmonize requirements where feasible to reduce burden, while maintaining the highest standards of care and use for regulated animals used in research.
Let’s look at where we are going:
The Guide for the Care and Use of Laboratory Animals outlines specific animal care requirements, as well as some deviations from these requirements. These deviations, referred to as departures, have specific reporting requirements. We are currently analyzing the feedback we received from you in response to an RFI on departures from the Guide, which will be very helpful in how OLAW will clarify these requirements going forward.
Other RFIs seeking your comments are expected in the future, each with at least sixty days to respond, around the following areas:
- Flexibilities for conducting program review
- Streamlining protocol review
- Exemptions from IACUC review
Let’s look at how you, your institution, and IACUC can be more involved:
We’ve teamed up with the Federal Demonstration Partnership and the IACUC Administrators Association on several joint initiatives to reduce burdens associated with laboratory animal programs. For instance, an online, open-access repository is under development to share standard procedures used in animal research protocols. A Universal IACUC Protocol Template that streamlines the IACUC application to be more user friendly while satisfying regulatory requirements is in the works. And to know if our efforts were effective, we are also planning a survey to get your thoughts.
Much is still to be done. We look forward to your continued participation and constructive input along the way.
Want more? Consider signing up for OLAW’s ListServ to receive information as it is announced.



0 Comments